In The Hemoglobin Buffer System
In the hemoglobin buffer system. As the red cell circulates. In this system hemoglobins migrate only partly due to their. Many commercial products are.
Hemoglobin S is more positively charged than Hb A and hence has a different electrophoretic mobility. In agar gel using an acetic acid-acetate buffer at an acid pH 60. Erythrocyte ĕ-rithro-sīt one of the formed elements in the peripheral blood constituting the great majority of the cells in the blood.
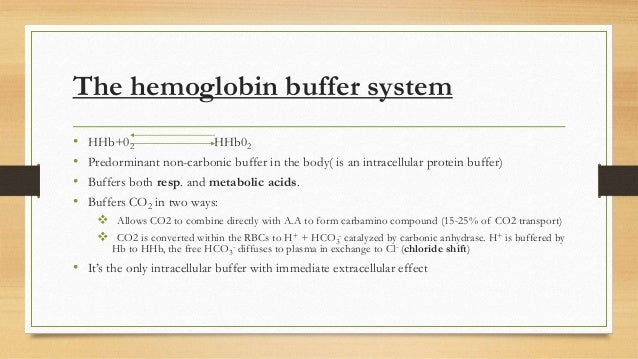
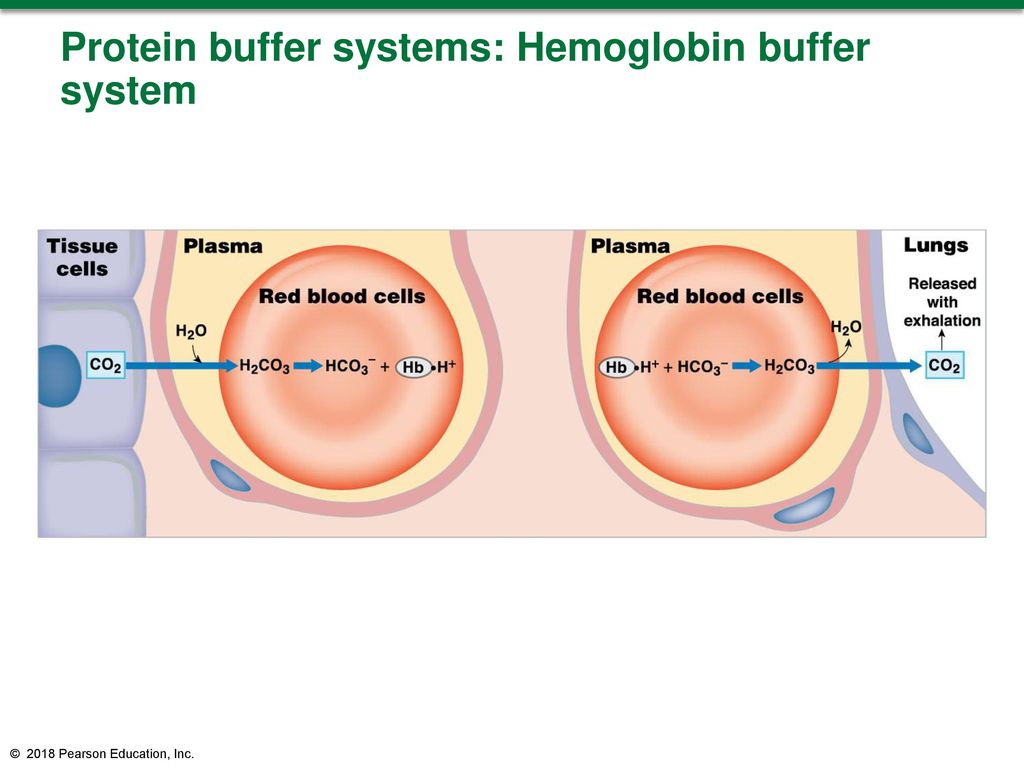
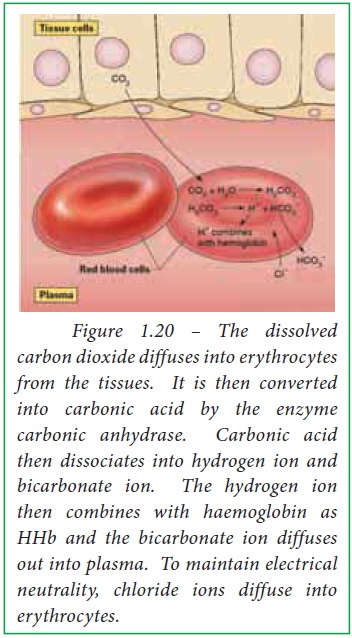
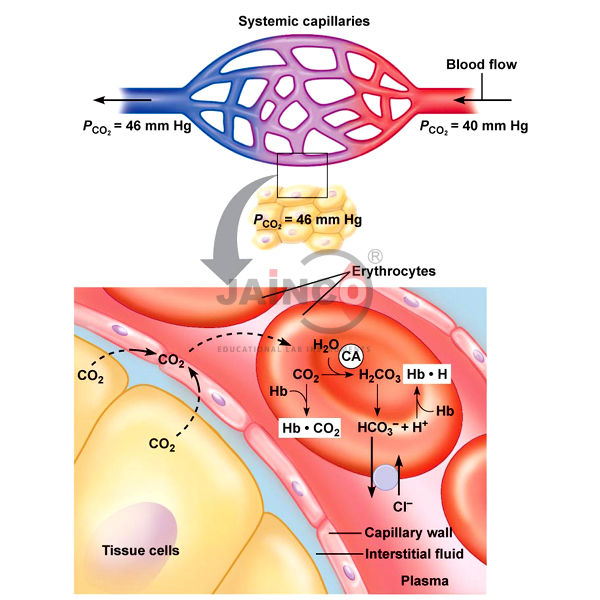
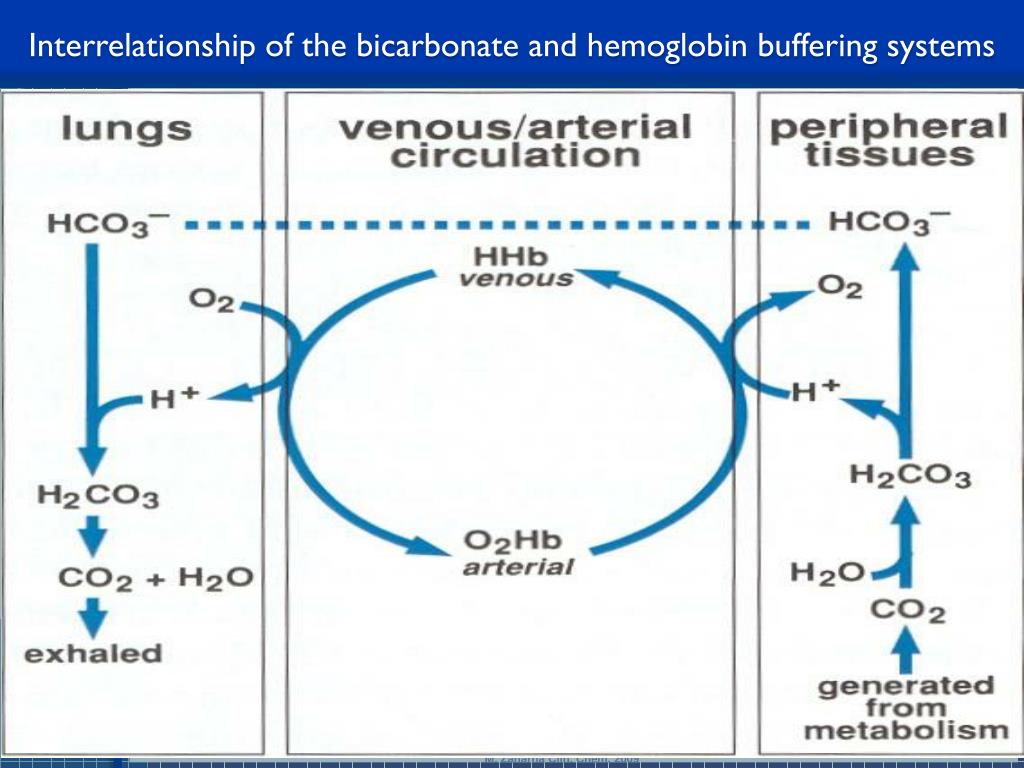
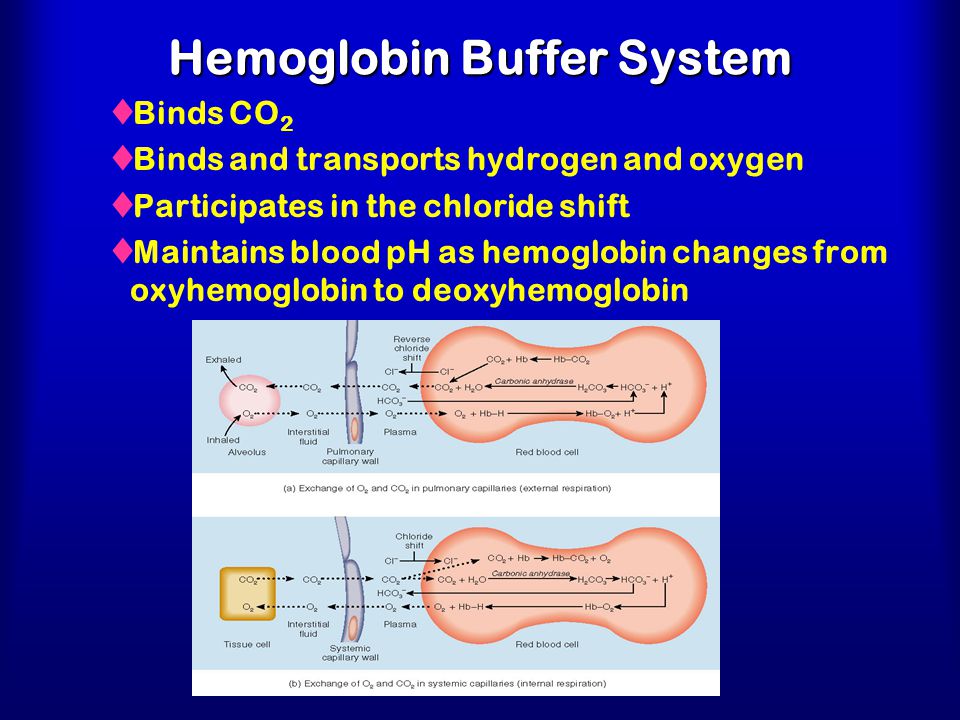
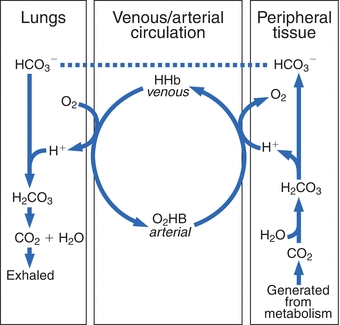
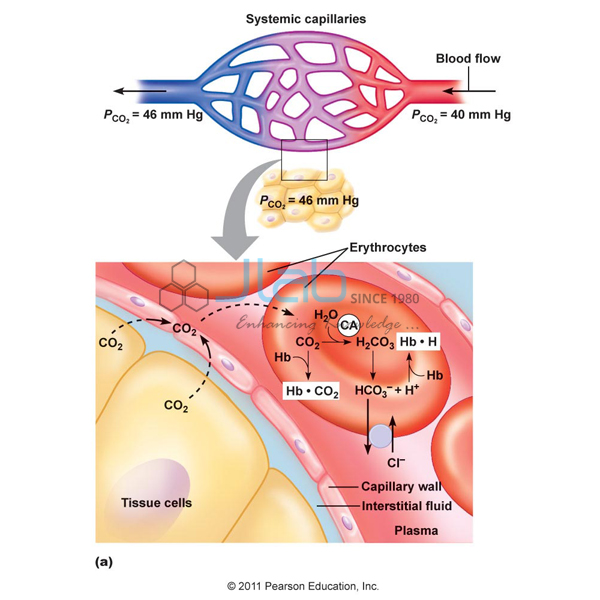
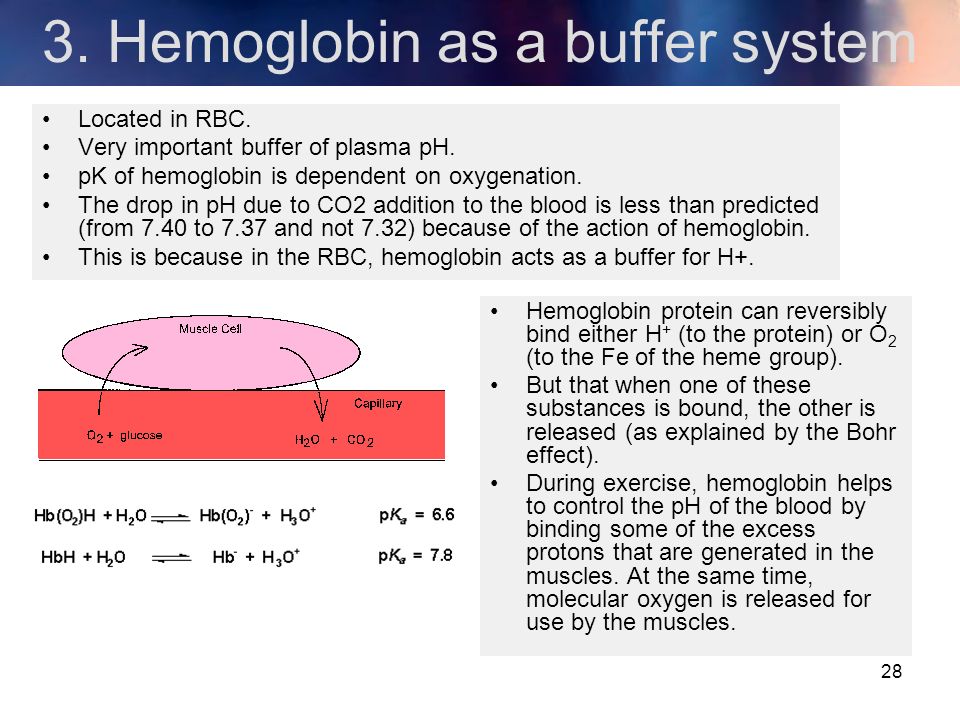
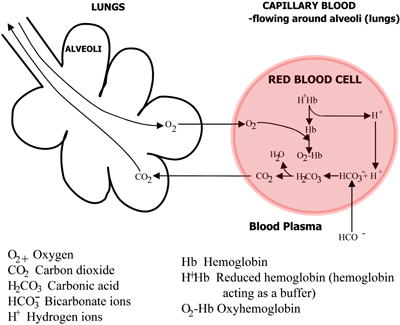
Binding of carbon dioxide to hemoglobin is reversible. This buffering helps maintain normal pH. For immature forms see erythrocytic series In humans the normal mature erythrocyte is a biconcave disk without a nucleus about 77 micrometers in diameter consisting mainly of hemoglobin and a supporting.
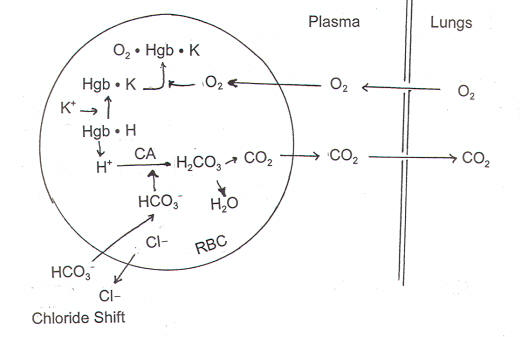
Deoxygenated hemoglobin has a higher affinity for CO 2 because it is a better proton acceptor than oxygenated hemoglobin. Thus the descriptive term respiratory alkalosis. Hemoglobin is the principal protein inside of red blood cells and accounts for one-third of the mass of the cell.
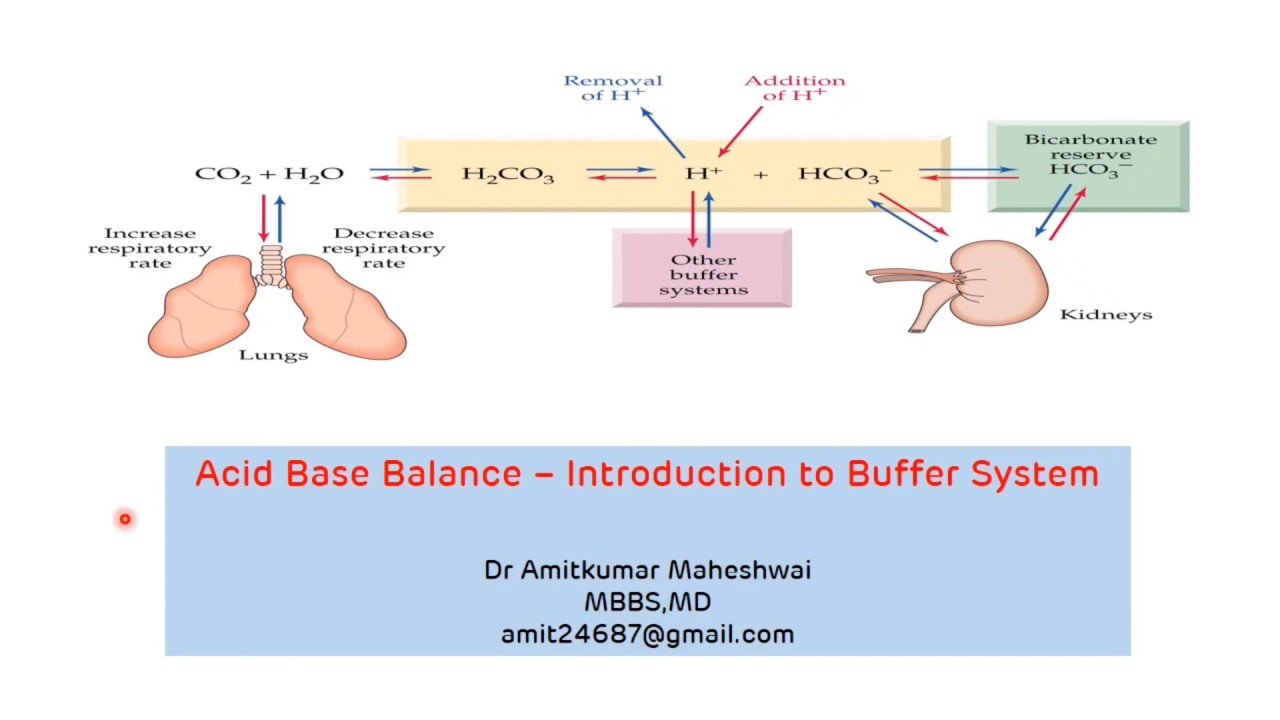
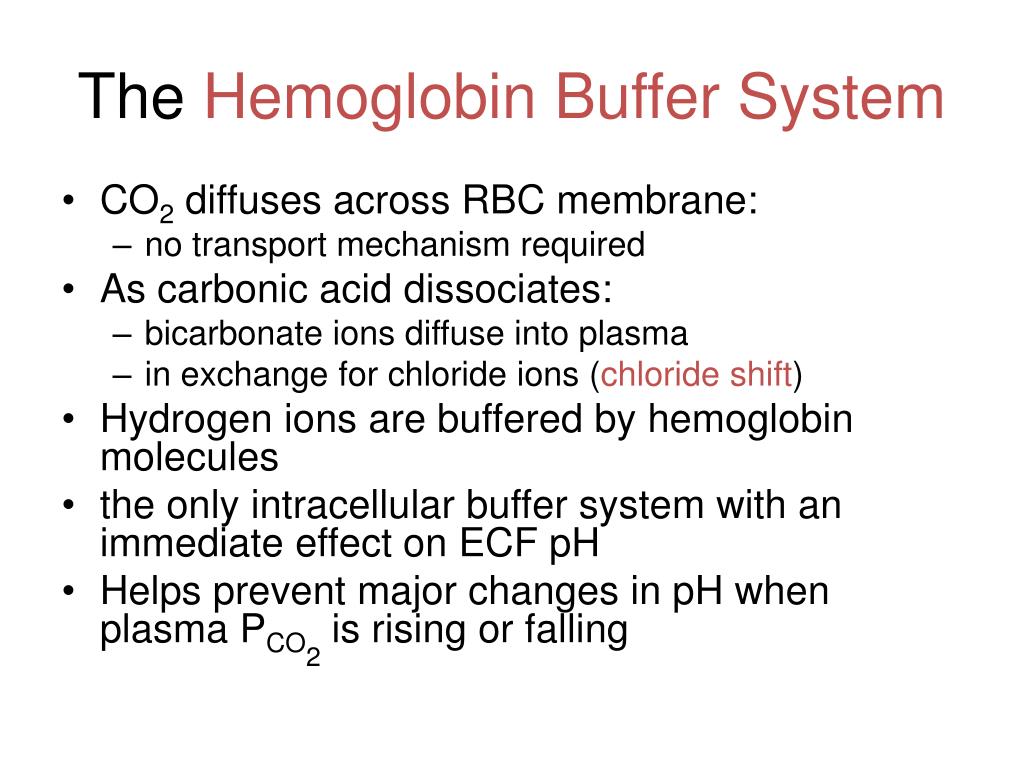
Both fetal and adult hemoglobin have four subunits but two of the subunits of fetal hemoglobin have a different structure that causes fetal hemoglobin to have a greater affinity for oxygen than does adult hemoglobin. The bicarbonate buffering system maintains optimal pH levels and regulates the carbon dioxide concentration that in turn shifts any acidbase imbalance. In this system hemoglobins migrate according to their charge as shown in the diagram.
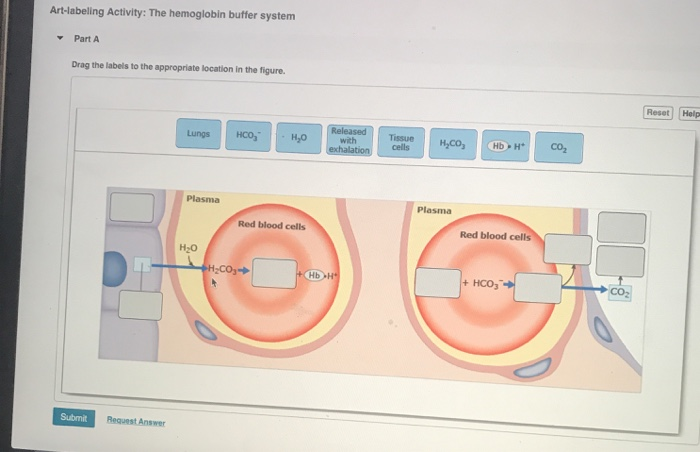
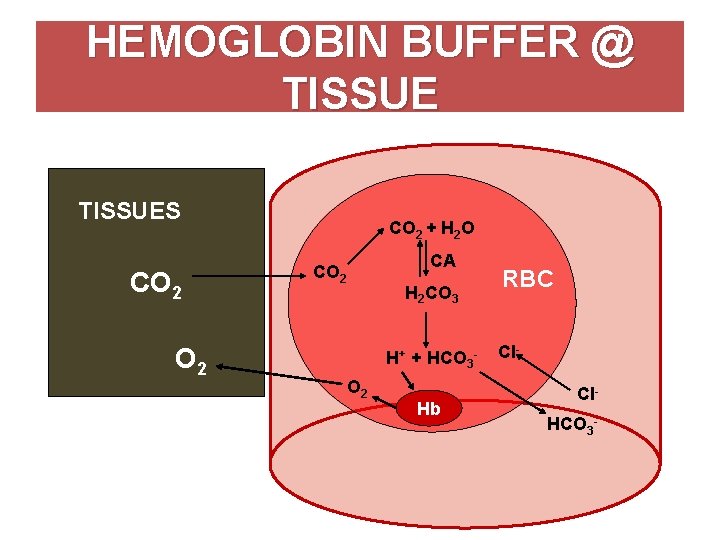
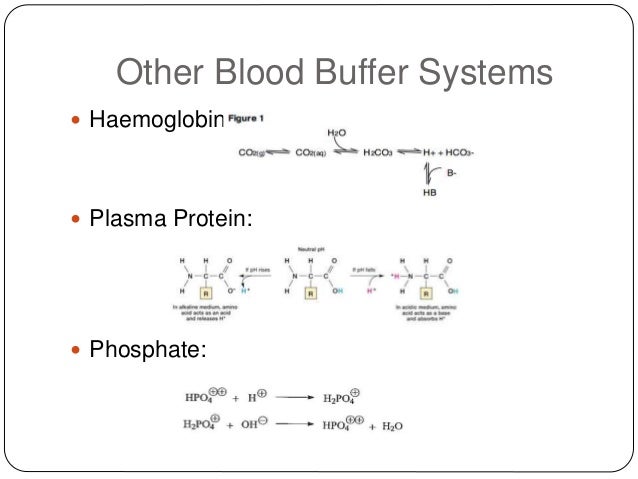
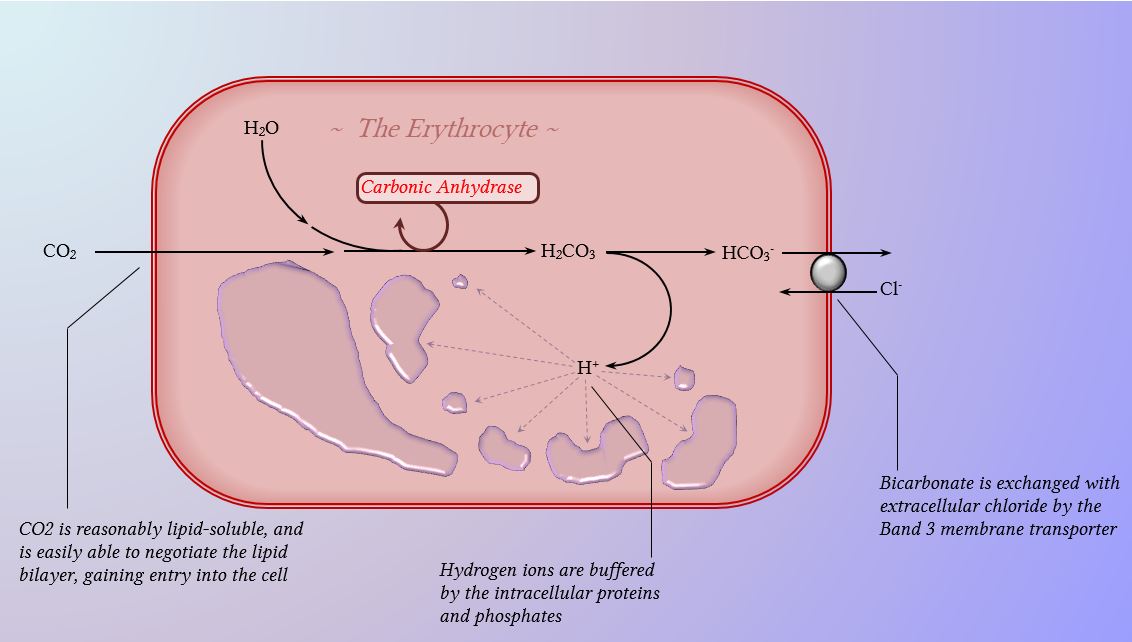
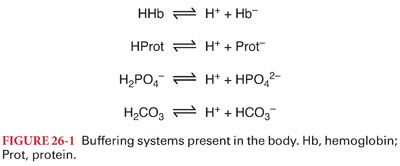
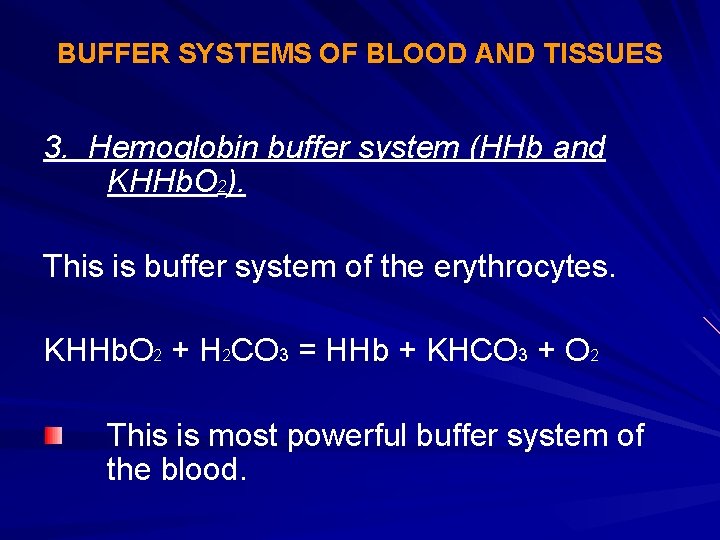
The components of this system are CO 2 HCO 3. Hemoglobin as a Buffer. And the word buffer in our everyday language it refers to something that kind of smooths the impact of something or it reduces the shock of something.
H_3O HCO_3- rightleftharpoons H_2CO_3 H_2O. Quaternary structure of hemoglobin.
Hayashi A Suzuki T Shin M 1973 An enzymic reduction system for mode.
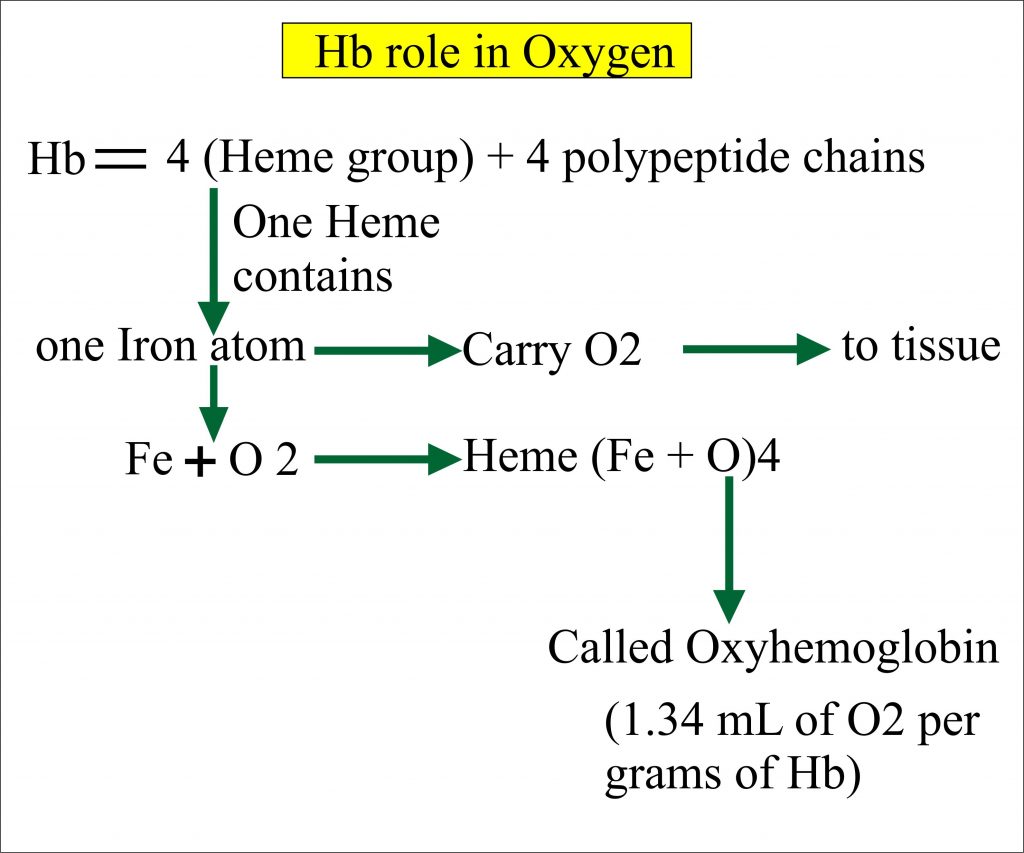
The oxyhemoglobin dissociation curve is a vital tool for comprehending how blood transports and releases oxygen. In this system hemoglobins migrate only partly due to their. The carbon dioxide produced as a result of metabolism reacts with water to form carbonic acid. Oxygen is carried throughout the body primarily by a protein molecule hemoglobin which is present inside red blood cells. This buffering helps maintain normal pH. Na 2 HPO 4. Many commercial products are. Both fetal and adult hemoglobin have four subunits but two of the subunits of fetal hemoglobin have a different structure that causes fetal hemoglobin to have a greater affinity for oxygen than does adult hemoglobin. Deoxygenated hemoglobin has a higher affinity for CO 2 because it is a better proton acceptor than oxygenated hemoglobin.
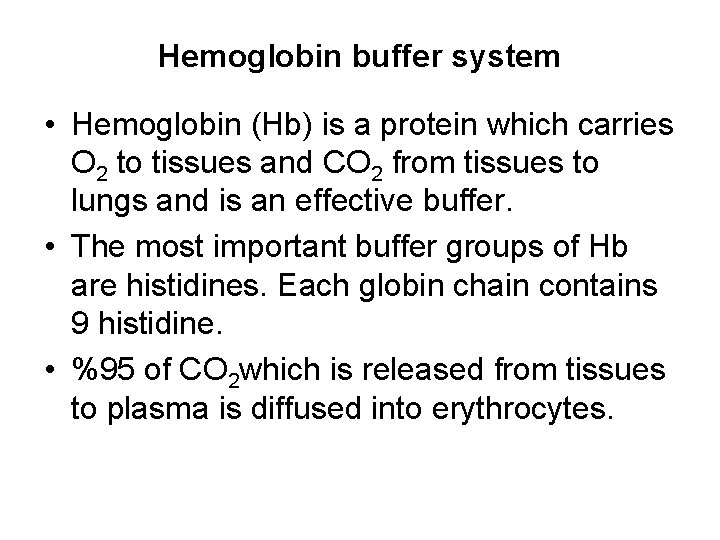
Kitagawa T 1988 The heme protein structure and the iron histidine stretching 29. Kohne 2011These mutations are broadly subdivided into those that impair globin protein subunit production thalassemias and those that produce structurally abnormal globin proteins Hb variants. This system continues during exercise providing continuous oxygen to working tissues. For immature forms see erythrocytic series In humans the normal mature erythrocyte is a biconcave disk without a nucleus about 77 micrometers in diameter consisting mainly of hemoglobin and a supporting. Hemoglobin also acts as a pH buffer in the blood. In this system hemoglobins migrate only partly due to their. An increase in hydronium causes this equilibrium to shift towards the oxygen side thus releasing oxygen from hemoglobin molecules into the surrounding tissuescells.




















Post a Comment for "In The Hemoglobin Buffer System"