Bethesda System For Reporting Thyroid Cytopathology
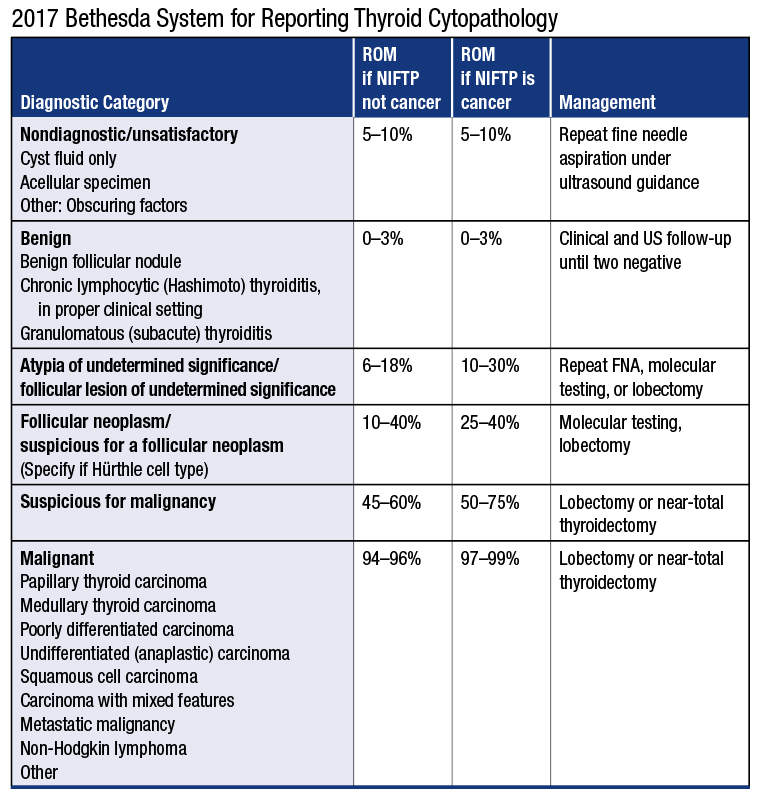
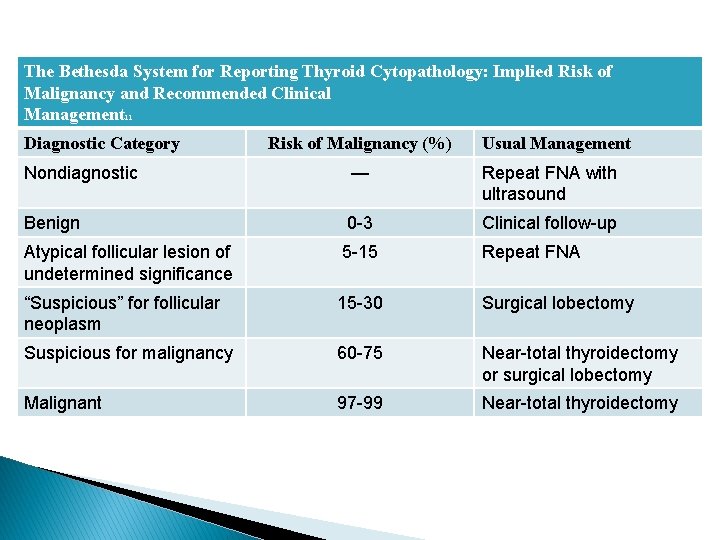
Bethesda system for reporting thyroid cytopathology. The Bethesda System for Reporting Thyroid Cytopathology TBSRTC is the offspring of the 2007 National Cancer Institute State of Science Conference on thyroid FNA that defines consensus diagnostic terminology and morphologic criteria. 58 TBSRTC provides uniform diagnostic terminology for pathologists to communicate with clinicians. The conclusions of the meeting led to the Bethesda Thyroid Atlas Project and formed the framework for The Bethesda System for Reporting Thyroid Cytology TBSRTC.
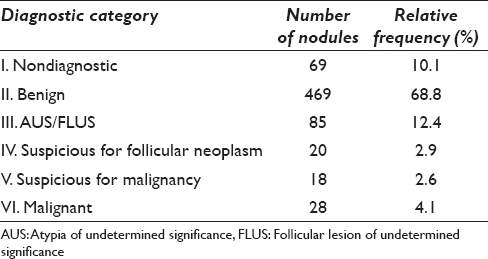
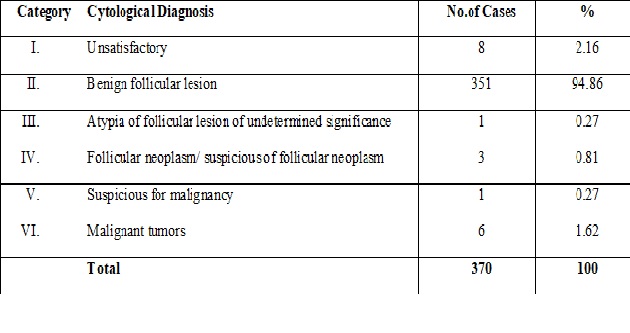
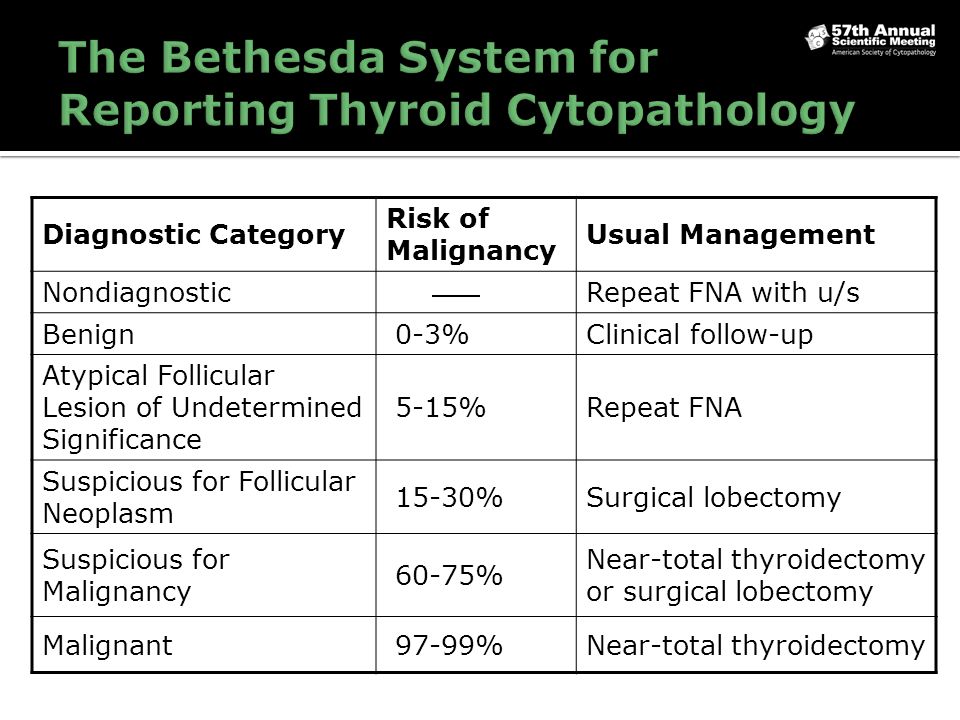
Consecutive thyroid fine-needle aspirates patient age 18 years were retrospectively collected from 7 tertiary centers in 5 Asian countries. Each of the categories has an implied cancer risk that links it to an appropriate clinical management guideline. Understandable by various specialists in different countries.
Ad See the Reporting Systems your competitors are already using - Start Now. Fadi Brimo Cheng Wang Harman Sekhon Omar Al-Nourhji Gabor Fischer Cady Zeman-Pocrnich January 25 2019 The CSC endorses The Bethesda System for Reporting Thyroid Cytopathology 2017. The Bethesda System for Reporting Thyroid.
It is a rapid cost-effective and very useful method in classifying thyroid nodules as either benign nodules reducing unnecessary surgery or malignant nodules requiring surgery123456 The Bethesda System for Reporting Thyroid Cytopathology TBSRTC was introduced in 2007 to standardize terminology used in reporting thyroid cytology. The recent update of The Bethesda System for Reporting Thyroid Cytology TBSRTC is a very important development in the evaluation of thyroid nodules. It is based on the NCI classification of 2008.
The Bethesda System for Reporting Thyroid Cytopathology TBSRTC established a standardized category-based reporting system for thyroid fine-needle aspiration FNA specimens. The adoption of the system will facilitate communication among the cytopathologist surgeon endocrinologist and radiologist and also allow easy and reliable sharing of data from different laboratories for national and. W ith its inception The Bethesda System for Reporting Thyroid Cytopathology TBSRTC established a standardized reporting system with a limited number of diagnostic categories for thyroid fine-needle aspiration FNA specimens.
Karger Medical and Scientific Publishers JAYPEE BROTHERS PUBLISHERS Wolters Kluwer India Pvt Ltd Springer Springer Science Business Media Jones Bartlett Publishers Elsevier Health Sciences Springer Nature Springer-Verlag John Wiley Sons. Contents Introduction Bethesda categories Nondiagnostic or Unsatisfactory Benign Atypia of Undetermined Significance or Follicular Lesion of Undetermined Significance Follicular Neoplasm or Suspicious for a Follicular Neoplasm Suspicious for. The Bethesda System For Reporting Thyroid Cytopathology Definitions Criteria And Explanatory Notes 2010 Edition Published By Springer 2009 published by.
The terminology proposed and illustrated in this text has been widely adopted not only in the US. The first edition of The Bethesda System for Reporting Thyroid Cytopathology was published in 2010 and has greatly influenced the practice of thyroid cytopathology.
W ith its inception The Bethesda System for Reporting Thyroid Cytopathology TBSRTC established a standardized reporting system with a limited number of diagnostic categories for thyroid fine-needle aspiration FNA specimens.
The Bethesda System for Reporting Thyroid Cytopathology Summary provided by Dr. The Bethesda System for Reporting Thyroid Cytopathology Moderator Dr G K Parvathidevi Presenter Dr Dhanya A N 2. The recent update of The Bethesda System for Reporting Thyroid Cytology TBSRTC is a very important development in the evaluation of thyroid nodules. The adoption of the system will facilitate communication among the cytopathologist surgeon endocrinologist and radiologist and also allow easy and reliable sharing of data from different laboratories for national and. Uniform terminology aimed to standardize the reporting of thyroid fine needle aspiration FNA cytology. A report of 2781 cases in a Chinese population. The Bethesda System for Reporting Thyroid Cytopathology TBSRTC is the offspring of the 2007 National Cancer Institute State of Science Conference on thyroid FNA that defines consensus diagnostic terminology and morphologic criteria. W ith its inception The Bethesda System for Reporting Thyroid Cytopathology TBSRTC established a standardized reporting system with a limited number of diagnostic categories for thyroid fine-needle aspiration FNA specimens. It is based on the NCI classification of 2008.
These reporting systems provide a uniform approach for classifying and reporting cytology in. The 2017 revision reaffirms that every thyroid FNA report should begin with 1 of 6 diagnostic categories the names of which remain unchanged since they were first. Contents Introduction Bethesda categories Nondiagnostic or Unsatisfactory Benign Atypia of Undetermined Significance or Follicular Lesion of Undetermined Significance Follicular Neoplasm or Suspicious for a Follicular Neoplasm Suspicious for. The adoption of the system will facilitate communication among the cytopathologist surgeon endocrinologist and radiologist and also allow easy and reliable sharing of data from different laboratories for national and. The Bethesda System for Reporting Thyroid Cytopathology TBSRTC. The Bethesda System for Reporting Thyroid Cytopathology TBS is an international reporting system for thyroid cytology. The first edition of The Bethesda System for Reporting Thyroid Cytopathology was published in 2010 and has greatly influenced the practice of thyroid cytopathology.







































Post a Comment for "Bethesda System For Reporting Thyroid Cytopathology"